Metabolischer Crosstalk bei Krebserkrankungen
Prof. Dr. Christiane Opitz
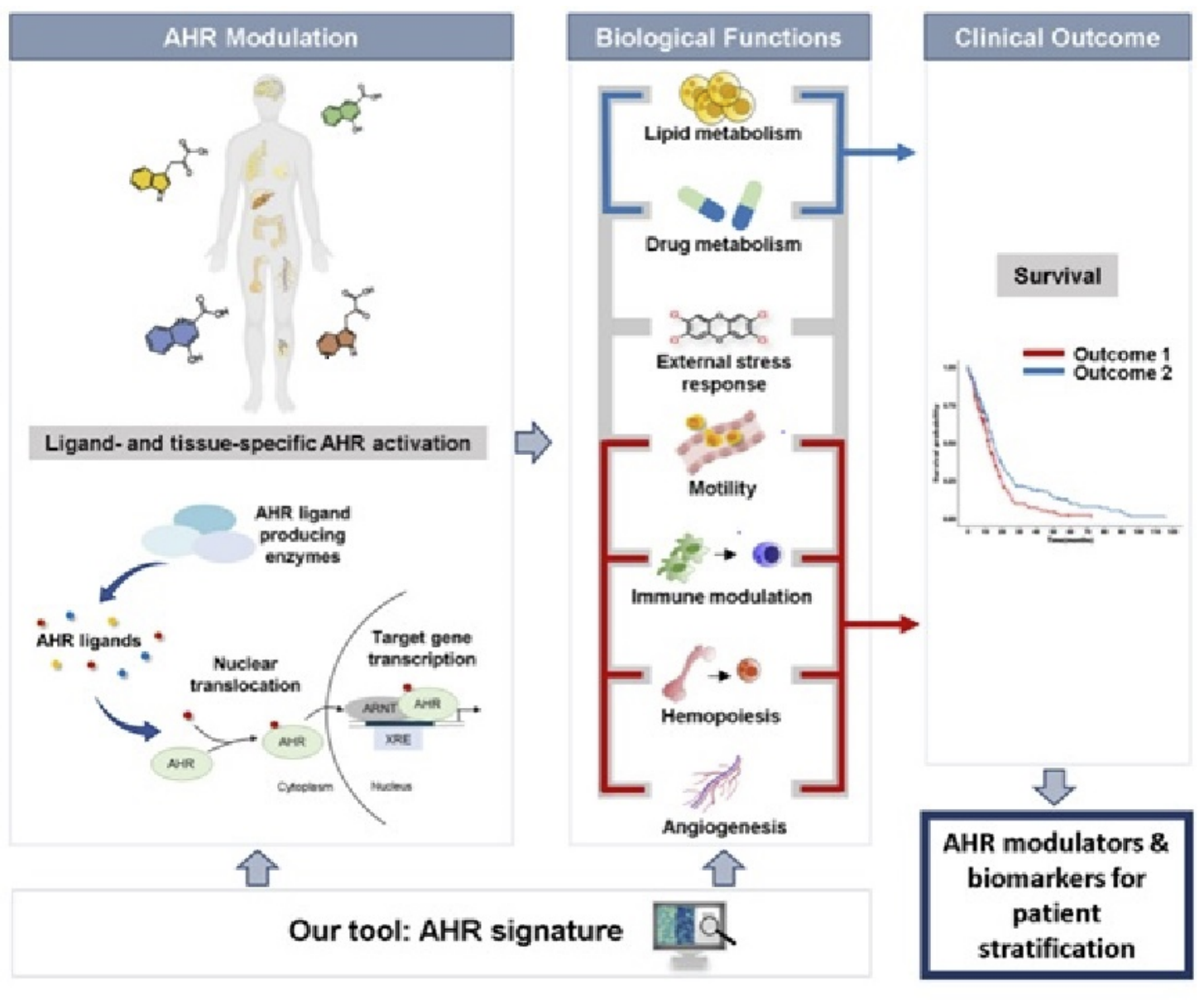
Das Hauptziel der Abteilung „Metabolischer Crosstalk bei Krebserkrankungen“ besteht darin, zu verstehen, wie Metabolite als Signalmoleküle im Austausch zwischen Tumorzellen und Tumormikroumgebung wirken und so zu malignen Eigenschaften von Tumorzellen und/oder zur Unterdrückung anti-tumoraler Immunantworten beitragen. Diese Interaktion ist entscheidend, da sie sowohl die maligne Transformation und Progression von Tumorzellen als auch deren Fähigkeit zur Immunevasion prägt. Das Verständnis der metabolischen Signalwege in diesem Austausch eröffnet neue Ansatzpunkte zur Identifizierung metabolischer Verwundbarkeiten in Tumoren.
Unsere Grundlagenforschung hat zur mechanistischen Identifikation metabolischer Biomarker geführt, insbesondere solcher, die mit Tryptophan, Tryptophan-Metaboliten, Aminosäuren, Aminosäure-Metaboliten und Ceramiden verknüpft sind. Diese Biomarker könnten zur Diagnose, Risikobewertung und zur Vorhersage des Therapieansprechens bei Krebspatientinnen und -patienten beitragen. Unser zentrales Ziel besteht darin, diese Biomarker zu validieren und die klinischen Szenarien zu definieren, in denen sie den größten Nutzen bieten. Der Nachweis ihres klinischen Mehrwerts stärkt präzisionsmedizinische Ansätze und ermöglicht langfristig ihre routinemäßige Anwendung in diagnostischen und prognostischen Abläufen, um eine stärker personalisierte und wirksamere Krebsbehandlung zu unterstützen.
Parallel dazu hat unsere Forschung neue metabolische Zielstrukturen für die Krebsimmuntherapie identifiziert. Diese Stoffwechselregulatoren und Enzyme beeinflussen die Funktion von Immunzellen und die anti-tumorale Immunität und eröffnen vielversprechende therapeutische Möglichkeiten. Wir entwickeln spezifische niedermolekulare Inhibitoren und zielgerichtete Protein-Degrader, um diese neuen Zielstrukturen gezielt zu hemmen. Die Wirkstoffkandidaten werden derzeit hinsichtlich Spezifität, Wirksamkeit und translationalem Potenzial optimiert, mit dem Ziel, sie in die klinische Entwicklung zu überführen.
Insgesamt ist unser Forschungsprogramm stark translational ausgerichtet und verbindet grundlegende mechanistische Erkenntnisse mit ihrer klinischen Anwendung. Durch den Fokus auf Krebsstoffwechsel und Immunmetabolismus entwickeln wir innovative Ansätze, die das Verständnis der Tumorbiologie vertiefen und gleichzeitig Diagnose, Prognose und Therapie von Krebspatientinnen und -patienten verbessern. Unser langfristiges Ziel ist es, metabolische Verwundbarkeiten in konkrete, klinisch nutzbare Strategien zu überführen, die effektivere und dauerhaftere anti-tumorale Therapien ermöglichen.
Zukünftige Projekte und Ziele
Unsere zukünftige Forschung konzentriert sich auf die Validierung metabolischer Biomarker für den Einsatz in der Krebsdiagnostik, der Risikostratifizierung und der Vorhersage des Therapieansprechens in unterschiedlichen klinischen Kontexten. Wir definieren die klinischen Anwendungen, in denen diese Marker den größten Nutzen erzielen und die Behandlungsergebnisse verbessern. Zudem treiben wir die Entwicklung „Small Molecule Inhibitors“ und „Degrader“ voran, die neue immunmetabolische Regulatoren adressieren und die anti-tumorale Immunität stärken sollen. Ein zentrales Ziel besteht darin, diese Erkenntnisse in klinisch nutzbare Werkzeuge und Therapien zu überführen und so die Verbindung zwischen Grundlagenforschung und Patient:innenversorgung weiter zu stärken.
