T-Zell-Antigen-Erkennung bei Krebs und Autoimmunität
Prof. Dr. Johannes Huppa
Unser Forschungsschwerpunkt liegt darauf, wie die adaptive und die angeborene Immunität zusammenwirken, um zwischen „selbst“, „fremd“ und „bösartig“ zu unterscheiden. Unsere Forschung konzentriert sich auf drei zentrale Fragestellungen:
- Wie erkennen T-Zellen Antigene – auf zellulärer, subzellulärer und molekularer Ebene?
- Welches Maß an Kreuzreaktivität zeigen T-Zellen beim Scannen einer Vielzahl unterschiedlicher Peptid/MHC-Komplexe mit geringer Affinität?
- Wie beeinflusst die strukturelle Degeneration (d. h. die strukturelle Vielfalt bei gleichzeitiger Funktionserhaltung) der T-Zell-Antigenerkennung die Immunantwort gegenüber Tumoren und Autoimmunerkrankungen?
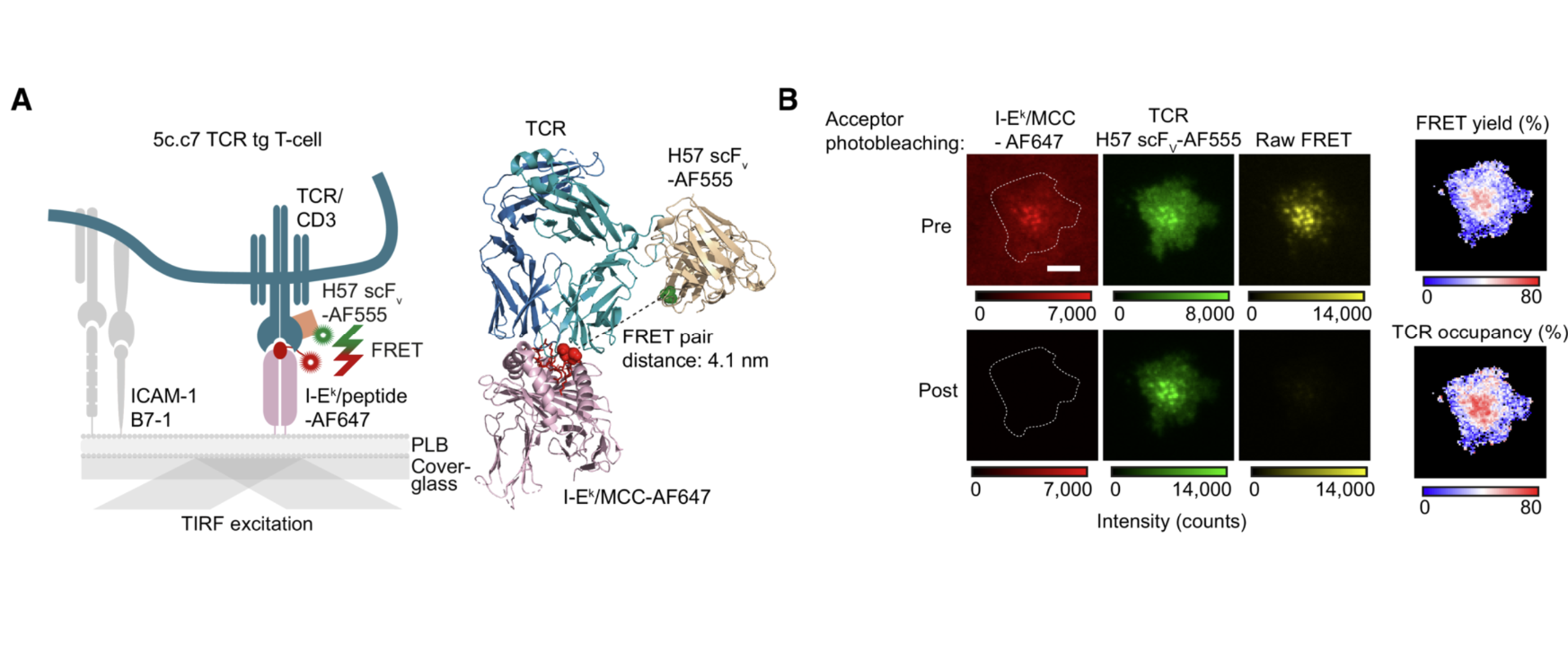
Unser Ziel sind quantitative Antworten unter Anwendung einer Kombination aus zellbiologischen, biophysikalischen, genetischen und Omics-basierten Methoden. Wir möchten verstehen, wie T-Zellen ihre bemerkenswerte Antigenselektivität und -sensitivität erreichen und dabei das empfindliche Gleichgewicht zwischen Toleranz und Immunität aufrechterhalten – ein Mechanismus, den wir als entscheidend für eine wirksame Anti-Tumor-Immunantwort betrachten. Die gewonnenen Erkenntnisse sollen genutzt werden, um T-Zell-basierte Präzisionsmedizin in der Krebs- und Autoimmuntherapie weiterzuentwickeln.
Um dies zu erreichen, entwickeln und nutzen wir fluoreszierende Sonden sowie protein-funktionalisierte, planare Lipid-Doppelschichten, die es ermöglichen, primäre T-Zellen in Echtzeit mittels fortgeschrittener Einzelmolekül-Live-Cell-Imaging-Technologien zu beobachten. Künftig möchten wir zusätzlich Strukturbiologie für eine verbesserte räumliche Auflösung sowie maschinelles Lernen einsetzen, um die Komplexität der Membranbiophysik und Systembiologie im Zusammenhang mit T-Zell-Antigenerkennung und T-Zell-Immunität bei Krebs umfassend zu erfassen.
Zukünftige Projekte und Ziele
Wir möchten die Mechanismen aufdecken, die der außergewöhnlichen Sensitivität der T-Zell-Antigenerkennung zugrunde liegen und T-Zellen prinzipiell hochwirksam gegenüber Tumoren mit heterogener und dynamischer Antigenexpression machen. Um die räumliche Auflösung weiter zu verbessern, werden wir fortschrittliche Live-Cell-Mikroskopie mit Strukturbiologie kombinieren – mit besonderem Fokus auf die CD4- und CD8-Korezeptoren. Diese binden an MHC I bzw. MHC II und verstärken die Antigenerkennung um bis zu das 600-Fache.
Darüber hinaus möchten wir experimentelle und rechnergestützte prädiktive Methoden zur TCR-Deorphanisierung anwenden und weiterentwickeln, um die Zielantigenspezifität tumorinfiltrierender Lymphozyten zu identifizieren. Nach dem Antigen-Matching evaluieren wir T-Zell-Rezeptoren (TCRs) hinsichtlich ihrer Sensitivität für die Antigenerkennung und leiten daraus optimierte, gentechnisch veränderte TCRs mit verbesserter Ligandenbindung für effektive patientenspezifische TCR-T-Zelltherapien ab.
Schließlich möchten wir mit unserer Arbeit zu dem übergeordneten Ziel beitragen, hochfunktionelle TCRs für jedes beliebige Tumorepitop vorhersagen zu können.

Prof. Dr. Johannes Huppa
Abteilungsleiter – Institut für Immunologie
Charité – Universitätsmedizin Berlin
T-Zell-Antigen-Erkennung bei Krebs und Autoimmunität / T-cell Antigen Recognition in Cancer and Autoimmunity