Nicht-kodierende Krebsbiologie
Dr. Johannes C. Hellmuth
Das nicht-kodierende Genom spielt eine grundlegende Rolle bei menschlichen Erkrankungen, einschließlich Krebs. Unser Ziel ist es, ein tieferes Verständnis der regulatorischen Schaltkreise im Krebsgenom zu gewinnen, um spezifische Verwundbarkeiten im nicht-kodierenden Genom von Krebszellen zu identifizieren.
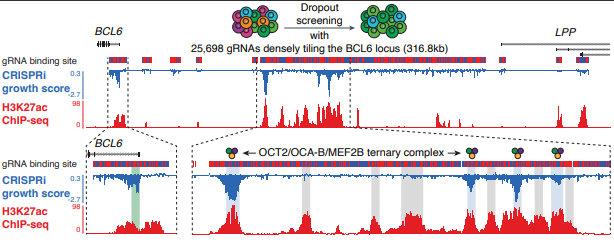
Derzeit liegt unser Fokus auf Super-Enhancern – großen regulatorischen Elementen im nicht-kodierenden Genom, die entscheidend zur Bestimmung der Zellidentität beitragen und eng mit onkogenen Funktionen bei Krebs assoziiert sind. Super-Enhancer unterscheiden sich in mehreren zentralen Aspekten von gewöhnlichen Enhancern und machen sie zu attraktiven therapeutischen Angriffspunkten: Sie kontrollieren Gene, die die Zellidentität definieren, sie sind stark zelltypspezifisch und sie sind auf transkriptionelle Kondensate angewiesen und dadurch besonders anfällig für Störungen.
Zukünftige Projekte und Ziele
- Funktionelle Untersuchung des nicht-kodierenden Genoms
Während das Repertoire essenzieller kodierender Gene bereits detailliert kartiert ist, fehlt ein vergleichbarer Ansatz für das nicht-kodierende Genom. Mithilfe groß angelegter CRISPRi-Screens identifizieren wir essenzielle Elemente im nicht-kodierenden Genom von Krebszellen und decken spezifische Verwundbarkeiten auf. - Super-Enhancer-Proteome
Obwohl die universell mit Super-Enhancern assoziierten Faktoren bekannt sind, ist unser Wissen über zelltypspezifische Transkriptionsfaktoren und Kofaktoren, die individuelle Super-Enhancer steuern, noch unvollständig. Wir nutzen verschiedene Screening-Strategien sowie massenspektrometriebasierte Verfahren, um die Proteome einzelner onkogener Super-Enhancer systematisch zu analysieren. - Super-Enhancer-Diagnostik
Super-Enhancer sind Schlüsselfaktoren der Zellidentität und bieten ein einzigartiges Fenster in die onkogenen Regulationsnetzwerke von Tumorzellen. Sie stellen eine funktional aussagekräftige Ebene des Epigenoms dar. Die Integration dieser Daten mit bestehenden Omics-Datensätzen wird unser Verständnis der Tumorbiologie vertiefen und präzisere, personalisierte Therapiestrategien ermöglichen. Wir planen daher die Entwicklung von Assays, mit denen sich die Aktivität von Super-Enhancern sowohl nicht-invasiv als auch in Tumorproben überwachen lässt.
