Onkologische Forschung
Prof. Dr. Sven Diederichs
Krebs wird häufig durch genetische Veränderungen wie Mutationen vorangetrieben. Für die überwiegende Mehrheit dieser Mutationen ist jedoch weder ihre funktionelle Relevanz für die Tumorentstehung noch ihr Einfluss auf das Therapieansprechen oder die Arzneimittelsensitivität bekannt. Dadurch bleibt ein großer Teil der Informationen aus der Krebsgenomanalyse ungenutzt und fließt nicht in klinische Entscheidungsprozesse ein.
Die funktionelle Genomik ist daher entscheidend, um Daten aus Krebsgenomen zu interpretieren und die biologische Bedeutung jeder einzelnen Mutation zu verstehen. Die Präzisionsonkologie beruht darauf, Mutationen zu charakterisieren, um Patient:innen gezielt mit den am besten geeigneten Therapien zu behandeln. Abgesehen von wenigen gut untersuchten Hotspot-Mutationen gelten jedoch die meisten Krebs-Mutationen als „variants of unknown significance“ (VUS), deren Einfluss auf Signalaktivierung sowie Arzneimittelsensitivität oder -resistenz weitgehend unbekannt ist. Dies schränkt die Zahl therapeutischer Optionen erheblich ein – selbst dann, wenn Inhibitoren für die entsprechenden Zielstrukturen verfügbar sind. Angesichts der Vielzahl an Mutationen in Krebsgenomen sind Hochdurchsatz-Methoden unverzichtbar, um relevante Mutationsmuster parallel charakterisieren zu können.
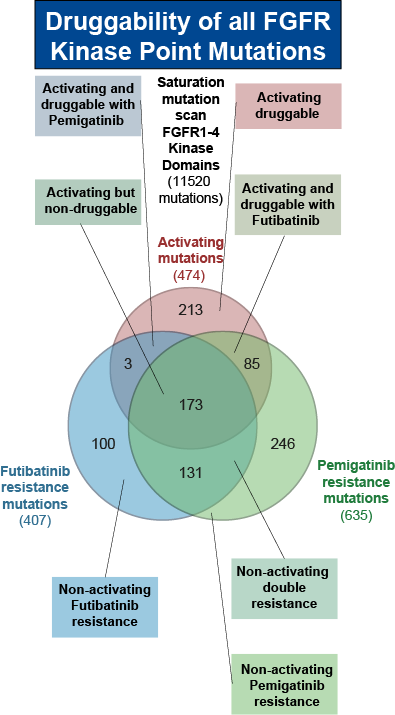
Unser Forschungsfokus liegt daher auf hochparalleler funktioneller und translationaler Genomik auf Mutations-Ebene. Wir untersuchen nicht nur die Wirkung kompletter Gene auf Krebs, sondern explizit den Effekt jeder einzelnen Mutation, da auch individuelle Tumoren nur eine bestimmte Kombination solcher Mutationen aufweisen. Ein zentrales Modell hierfür ist die Familie der Fibroblasten-Wachstumsfaktorrezeptoren (Fibroblast Growth Factor Receptors, FGFRs). Ihre Mitglieder FGFR1, FGFR2, FGFR3 und FGFR4 sind in zahlreichen Tumorarten verändert – unter anderem bei Lungen-, Blasen-, Brust- oder Endometriumkarzinomen sowie beim Cholangiokarzinom – und stellen etablierte Treiber der Tumorigenese dar. Für FGFR existieren zudem zugelassene Inhibitoren in der Klinik. Dennoch ist für die Vielzahl häufig vorkommender Punktmutationen unklar, ob sie den FGFR-Signalweg tatsächlich aktivieren und welchen Einfluss sie auf Medikamentensensitivität oder -resistenz haben.
Um diese Lücke zu schließen, haben wir eine umfassende Mutationsbibliothek erstellt, die jede mögliche Mutation der FGFR-Familie umfasst – insgesamt fast 30.000 Varianten. Diese testen wir systematisch auf Aktivierungspotenzial und Medikamenten-Targeting. Das daraus entstehende Katalogwerk funktioneller Mutationen ermöglicht es, Patient:innen gezielt für FGFR-Inhibitor-Therapien auszuwählen, bei denen ein Ansprechen wahrscheinlich ist, und gleichzeitig alternative Therapieoptionen für Patient:innen aufzuzeigen, deren Tumoren voraussichtlich nicht ansprechen.
Darüber hinaus identifizieren und charakterisieren wir nicht-kanonische Mutationen (EMBO Mol Med 2016), wie Nonstop-Extensions / Stop-Loss-Mutationen (Nat Cell Biol 2020) oder synonymen Mutationen (Nat Commun 2019), und untersuchen deren Rolle in der Krebsentstehung, ihre funktionelle Relevanz sowie die zugrundeliegenden molekularen Mechanismen.
Unser langfristiges Ziel ist es, eine funktionelle Charakterisierung einer Vielzahl von Krebs-Mutationen bereitzustellen, um sie für die Therapieentscheidung nutzbar zu machen.
Zukünftige Projekte und Ziele
In Zukunft werden wir unser FGFR-Saturation-Mutational-Scanning auf weitere FGFR-Inhibitoren ausweiten und anschließend auf andere Onkogene und Zielstrukturen übertragen, um die klinische Entscheidungsfindung zu unterstützen.
Für die klinische Translation unserer Ergebnisse planen wir, unsere Vorhersagen zur Arzneimittelsensitivität aus funktionellen Screenings in einer klinischen Studie zu überprüfen. Ziel ist es, eine vollständige Landkarte klinisch relevanter FGFR-Mutationen über verschiedene Tumorentitäten hinweg zu erstellen und so eine fundierte Basis für Präzisionstherapien zu schaffen.

Prof. Dr. Sven Diederichs
Abteilungsleiter – Partnerstandort Freiburg
Onkologische Forschung in der Thoraxchirurgie / Cancer Research in Thoracic Surgery