Contact CCP-Office
E-Mail: ccp@dkfz.de
Networked Biobanking within the BioDataHub
High-Quality bio samples – connected, accessible, research-driven
The networked biobanking infrastructure of the DKTK BioDataHub provides researchers with cross-site access to high-quality, well-characterized biospecimens from cancer patients, including associated clinical information from tumor documentation systems.
This comprehensive data foundation supports the planning and implementation of translational research projects and the development of new diagnostic and therapeutic approaches.
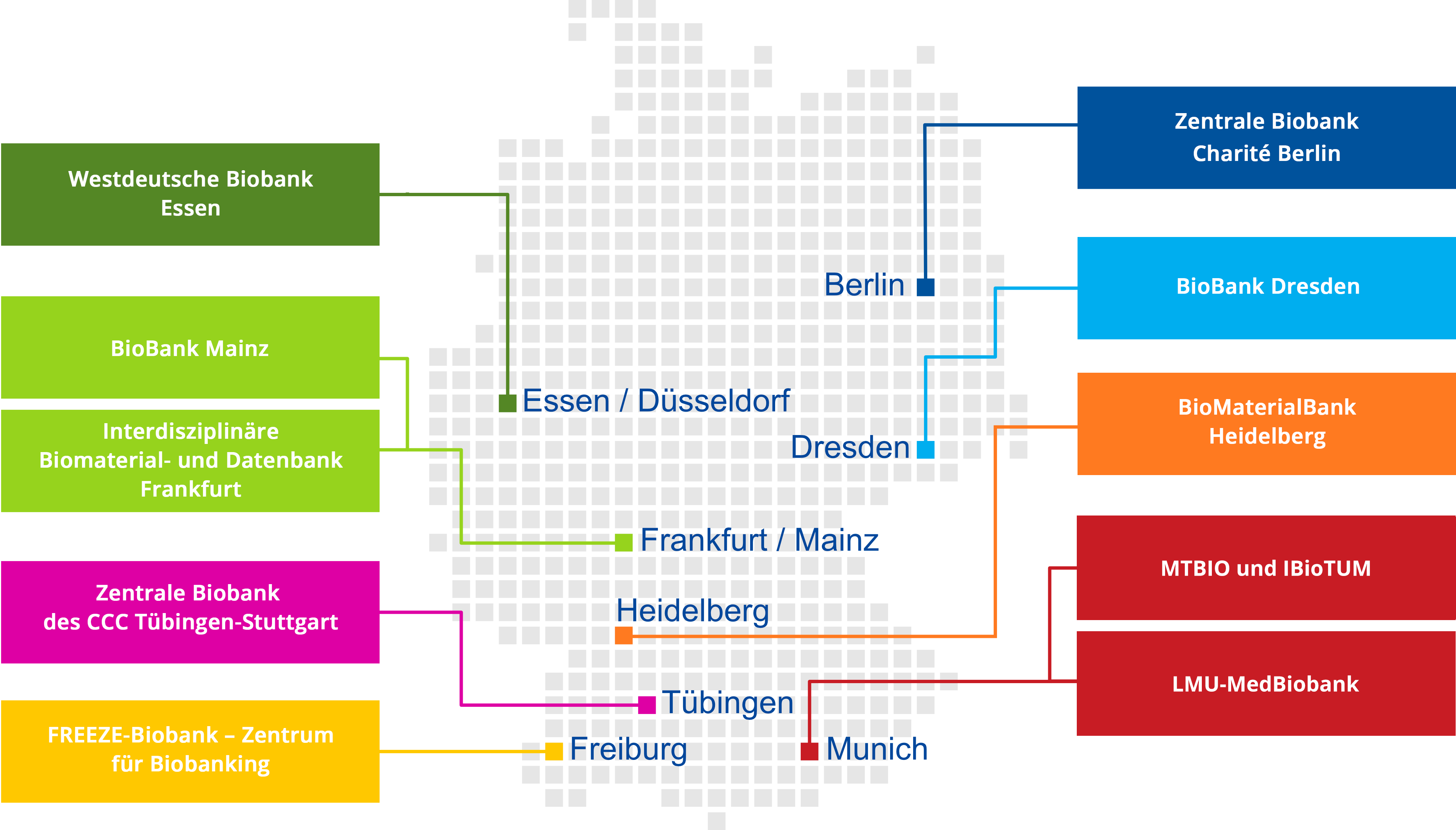
Connected infrastructure
The biobanks at the DKTK partner sites store tissue, blood, and liquid samples, as well as derived materials, in compliance with strict legal, ethical, and data protection requirements.
Through the BioDataHub, these biobanks are interconnected across the consortium:
Via the IT-Infrastruktur des BioDataHub bio sample information can be linked with clinical data from tumor documentation systems and made visible to researchers in the DKTK BioDataHub Explorer.
Supporting Research Projects
The BioDataHub provides researchers with guidance and support in planning biospecimen-based projects:
- Consultation on the collection, reuse, and documentation of biospecimens
- Referral to the centralized biobanks at the respective sites
- Support in developing scientific projects involving biospecimens
Quality Assurance and Biobank Collaboration
The BioDataHub ensures quality assurance and data harmonization across all participating biobanks. It safeguards compliance with ethical standards (e.g., informed patient consent) and data protection regulations and promotes collaboration with national initiatives such as the German Medical Informatics Initiative (MII) as well as international partners including BBMRI-ERIC.
Contact: For questions regarding the BioDataHub’s networked biobanks, please contact biodatahub@dkfz.de