The Clinical Communication Platform (CCP)
The DKTK Clinical Communication Platform (CCP) is an information hub for translational cancer research. It enables researchers to access patient data and biosamples from routine care and research across sites, provides patients with insight into ongoing clinical trials, and promotes networking within and beyond the consortium.
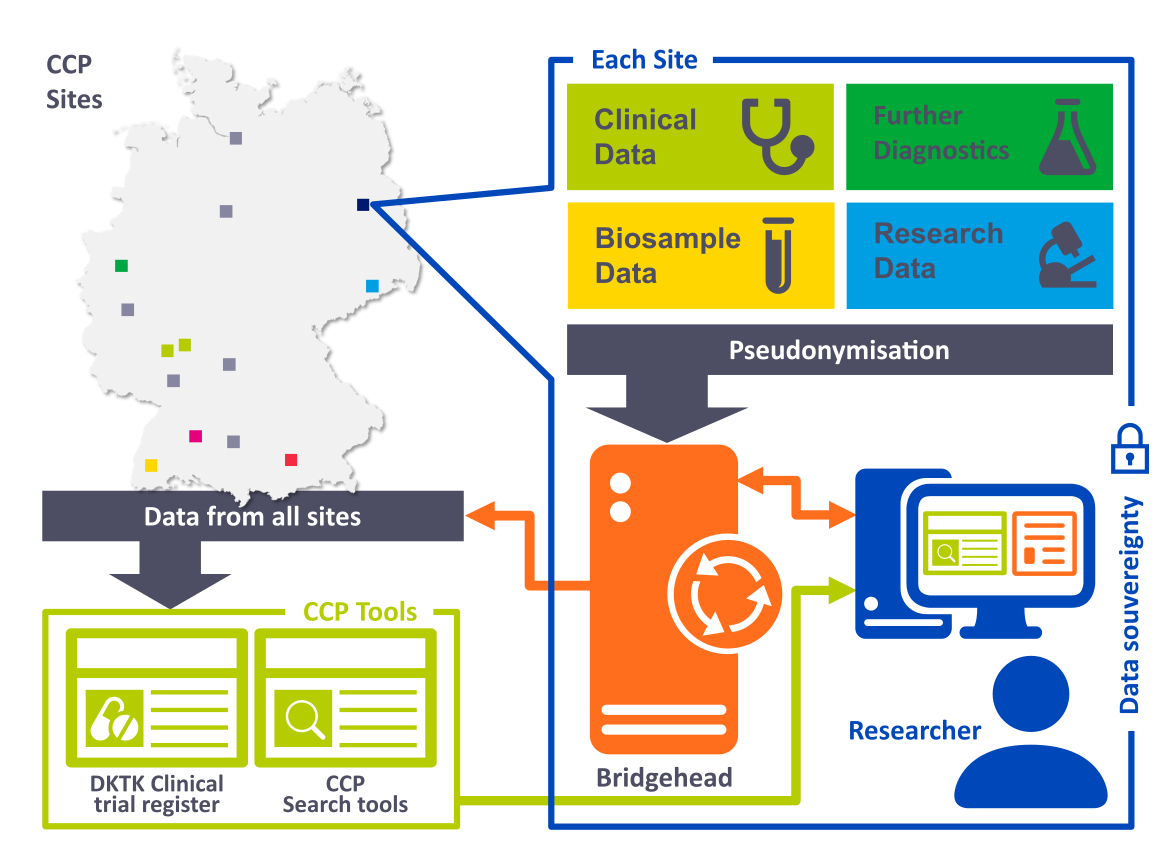
For the development and improvement of diagnostic procedures and personalized therapy of cancer diseases, scientists require sizable amounts of information. At DKTK and partner sites, the CCP acts therefore as a data bridge between clinical practice and research to provide researchers a network-wide access for the reuse of obtained information in compliance with data protection laws.
The federated infrastructure of the CCP with its technologies & IT solutions enables the integration of pseudonymised patient data from clinical care as well as research at each participating site and ensures availability for all beneficiaries. Networking with the biobanks at the sites complements the data on available biosamples and thus provides comprehensive information from clinical care for current and future research projects.
To empower researchers and their projects and to enable the reuse of data and biosamples for translational research in the network, the CCP offers a wide range of support to clinical researchers and DKTK Joint Funding projects. In addition, the CCP powers the DKTK Clinical Data Science Group to develop innovative use cases for oncology data and to expand interdisciplinary collaboration within the network.
From 2016 to 2025, the CCP has provided an overview and further information on clinical trials at all DKTK and partner sites for physicians, patients and patients’ relatives with the DKTK Clinical Trial Register. It was discontinued on August 31, 2025.
For further contact, please contact the CCP team.