02/11/2021
Print PageThe Proteomic Aspect of Tumor Profiling
Drugs already approved for the treatment of certain diseases can enable the treatment of other diseases through so-called "drug repurposing". These drugs have already been formally approved by the regulatory authorities. Therefore, compared to the development and approval of new drugs, the "Proof of Concept of Clinical Efficacy and Safety" for an alternative use can already be started with phase III clinical trials. Without phase I and II clinical trials, the time to regulatory approval of a drug is reduced from approximately 15 to 3 years.
The research group of B. Küster examined 243 clinically approved kinase inhibitors that block the growth of tumor cells (Klaeger et al, Science, 2017). As an example, he chose the drug cabozantinib, which is already used to treat thyroid cancer, renal cell carcinoma and hepatocellular carcinoma. It also has an effect on FLT3-ITD – the receptor tyrosine kinase FLT3 with a common driver mutation – in acute myeloid leukemia (AML) and could therefore play a potential role in the future treatment of AML. The freely accessible database www.ProteomicsDB.org, co-developed by B. Küster, shows a large collection of human and murine proteome data analyzed by mass spectrometry.
To predict the effectiveness of drugs on different types of cancer, B. Küster and his team investigated their effect on cancer cell lines. For example, they were able to explain the improved survival probability in breast cancer by a high phosphorylation rate of the progesterone receptor PGR (Frejno et al. NatCommun 2020).
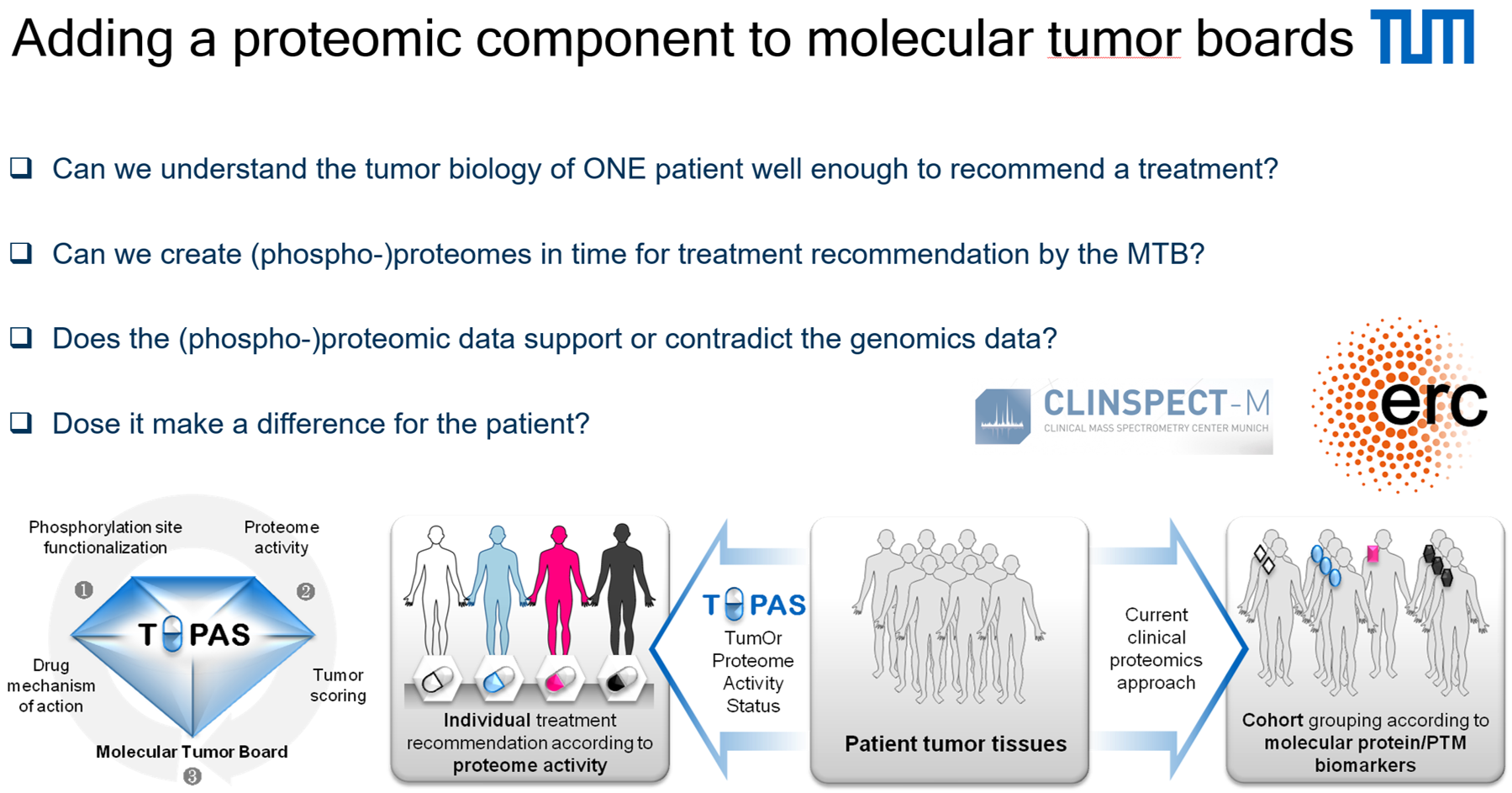
In his project TOPAS, B. Küster measures protein activity in cancer cells in the laboratory using quantitative mass spectrometry. Can this „TOPAS score“ provide clinicians with additional information about their patients? This question is currently being investigated in the molecular tumor boards, the interdisciplinary tumor conferences for consultation on the most promising therapy option for a patient: Here, for example, genomic data showed increased ABL1 expression, which could not be confirmed in the proteomic profile. To be able to interpret these differences in the data, medical and proteomic scientists are currently learning from each other. The objective is to be able to include these findings in the choice of treatment options in the future.
Anna Vetter, Scientific Coordinator DKTK Munich