05/02/2021
Print PageGenotoxic E. coli “caught in the act” of transforming primary colon cells
Colorectal cancer is the second most common cause of cancer-related deaths. Recent research findings indicate that the intestinal microbiome, i.e. the totality of microorganisms living in the intestine, plays an important role in the development and progression of cancer. For example, disturbances in the gut microbiome leads to inflammation, which contributes to cancer development. In addition, certain intestinal bacteria can directly damage the genetic material of intestinal cells with the help of so called genotoxins. They can alter DNA in such a way as to cause mutations that ultimately lead to cancer. A research team led by Thomas F. Meyer, senior professor at Kiel University (CAU), in cooperation with the group of Prof. Heiko Hermeking at the German Cancer Consortium (DKTK), partner site München, has now succeeded in catching a certain bacterium and its genotoxin "in the act". They observed that genotoxic E. coli induces genetic changes that are characteristic of colorectal cancer cells and cause a transformed phenotype – after only a few hours of infection. The team published their results today (Friday, Feb. 12, 2021) in the renowned journal Nature Communications.
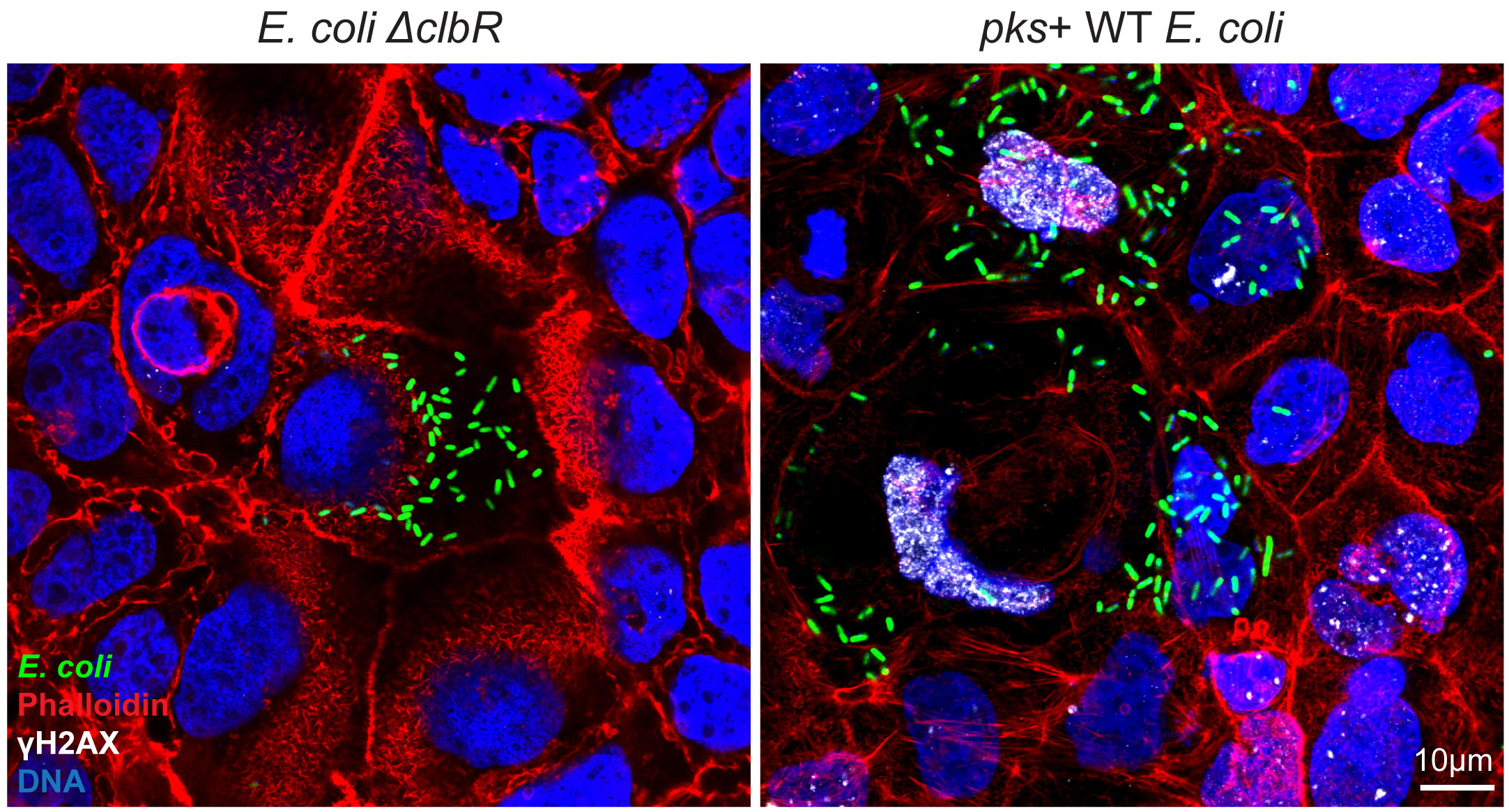
Escherichia coli bacteria (E. coli) are constitutive members of the human gut microbiota. However, some strains produce a genotoxin called colibactin, which is implicated in the development of colorectal cancer. More than two-thirds of colorectal cancer patients carry colibactin-producing E. coli strains in their gut and the number of carriers is rising in the western world. They are particularly prevalent in colorectal cancers associated with chronic intestinal inflammation such as Crohn's disease and ulcerative colitis. Epidemiological evidence for a link between certain bacterial species and some forms of human cancer abound – but it remains difficult to provide the direct proof required to justify extensive prevention strategies. The first clear evidence of a link between these bacterial strains and colorectal cancer was recently provided by Prof. Meyer, who is also a member of the Cluster of Excellence "Precision Medicine in Chronic Inflammation" (PMI), and his then team from the Max Planck Institute for Infection Biology in Berlin (MPIIB): The researchers identified the genetic signature colibactin leaves in host cells, and showed that it can be detected in a subgroup of colorectal cancers. Since such cancers take many years to develop, the actual process by which a normal cell becomes cancerous remained unclear.
In colon organoids, colibactin leads to uncontrolled proliferation
Now they have gone a significant step further by utilizing organoids to observe transformation itself. This new technology makes it possible to grow normal, primary colon epithelial cells in culture in the form of 3D spheres. These hollow “mini-organs” are generated by the adult stem cells that drive the rapid turnover of the colonic mucosa. Prior to the advent of this technology, infection experiments in vitro required cell lines, which are already partially transformed and thus unsuitable for recapitulating the very early stages of cancer development. To test whether colibactin-producing E. coli have any lasting effect on host cells, the team infected their organoids for three hours. This was already sufficient to induce changes that are characteristic of colorectal cancer.
"First, the cells began to multiply faster than normal - the best-known characteristic of cancer cells. What was particularly remarkable, however, was that after infection with colibactin-producing E. coli, some cells were able to survive without the growth factor "Wnt" in the culture medium, unlike normal stem cells," explains Prof. Meyer.
Stem cells have the potential to develop into a wide variety of cells in the body. Thus, new specialized intestinal cells are continuously formed from intestinal stem cells, which then take over digestive functions and do not proliferate any further. "In healthy tissue, the growth factor Wnt ensures that intestinal stem cells proliferate, so there are always enough of them to renew intestinal cells. As soon as the stem cells emerge from this Wnt-containing environment, they develop into specialized intestinal cells that do not proliferate further. This mechanism also prevents uncontrolled proliferation of the cells outside the Wnt-containing environment," explains the other lead author PD Dr. Michael Sigal, who recently founded his own research group on the topic at the Charité University Hospital in Berlin. However, if cells manage to proliferate independently of the Wnt signal, uncontrolled growth occurs, a precursor to cancer. The same phenomenon can be observed in the organoid: Organoid cells continuously require Wnt to proliferate; without this growth factor, they differentiate out just as they do in the healthy gut, transform into specialized cells, and die a while later. "However, when we transferred the E. coli-infected cultures to Wnt-free medium, a few cells survived and continued to form fast-growing organoids. Such growth factor independence is a typical feature of early colon cancer cells," said Amina Iftekhar, Ph.D., first author of the study and a former doctoral student at MPIIB.
Chromosomal changes possibly lead to cancer development
Next, the researchers analyzed the DNA of the organoid cells after treatment with colbactin-producing E. coli. Sequencing revealed that the genome contained numerous mutations, including large structural changes in the DNA: entire sections of chromosomes, the structures in which DNA strands are organized, had been gained, lost or rearranged. Such large chromosomal restructurings are found in most colon cancer cells. This observation could provide an important explanation for a previously unresolved issue: The specific genetic signature that colibactin leaves in intestinal cells, as Prof. Meyer and his team were able to show in the previous study, is found in only about 10% of colorectal cancer patients. This is despite the fact that most colorectal cancer patients carry colibactin-producing E. coli. "Accordingly, we would actually expect colibactin-induced genetic alterations to be present in significantly more colorectal cancer cases. And this is what our new results now indicate," explains Prof. Meyer.
Colibactin cross-links DNA strands at specific sites. The cell tries to repair this as precisely as possible with its repair mechanisms, and in some cases colibactin leaves its specific fingerprint in the DNA. "However, the precise repair often does not work; this is when botched repairs occur, leading to the gross chromosomal changes often observed in colorectal cancer. We suspect that these changes, which result from incorrect repair, form the actual basis for cancer development," Meyer continued.
"We do not yet know which factors influence how the cell tries to eliminate the linkage," says Prof. Meyer. In the future, he would like to investigate this in more detail in his newly founded research group "Infection Oncology" at the Institute for Clinical Molecular Biology at CAU and the University Hospital Schleswig-Holstein, together with the Cluster of Excellence PMI.
Among the genetic alterations induced by the bacteria were deletions of the gene that encodes the microRNA miR-34a and is inducible by p53. The functional consequences of the miR-34a inactivation were studied in a collaboration with the team of Prof. Heiko Hermeking at the Ludwig-Maximilians-Universität München (LMU) and German Cancer Consortium (DKTK), partner site München. The results show, that the loss of miR-34a contributes to the Wnt independence and thereby promotes uncontrolled proliferation of intestinal cells.
Original publication:
(1) Amina Iftekhar et al.: Genomic aberrations after short-term exposure to colibactin-producing E. coli transform primary colon epithelial cells. Nature communications (2021). https://doi.org/10.1038/s41467-021-21162-y
(2) Dziubańska-Kusibab PJ et al.: Colibactin DNA-damage signature indicates mutational impact in colorectal cancer. Nature Medicine (2020), https://doi.org/10.1038/s41591-020-0908-2
(Link to the press release of the Christian-Albrechts-Universität zu Kiel: https://www.precisionmedicine.de/en/details/news/genotoxic-e-coli-caught-in-the-act-of-transforming-primary-colon-cells)