07/10/2019
Print PageInfluential fusion partner of a new cancer-driver protein
BRAF is a human gene that encodes the BRAF serine/threonine kinase. The BRAF protein regulates important cell growth signals and its activity is strictly controlled, partly through its autoinhibitory ability and through its recruitment to the cell membrane by growth signals. Genetic modifications in a cell can lead to BRAF becoming a cancer gene: in this case, the growth signal is transmitted continuously and the cells start to multiply uncontrollably. BRAF mutations are found in many cancers, including lymphomas, colon cancer, skin cancer, lung cancer and brain tumors.
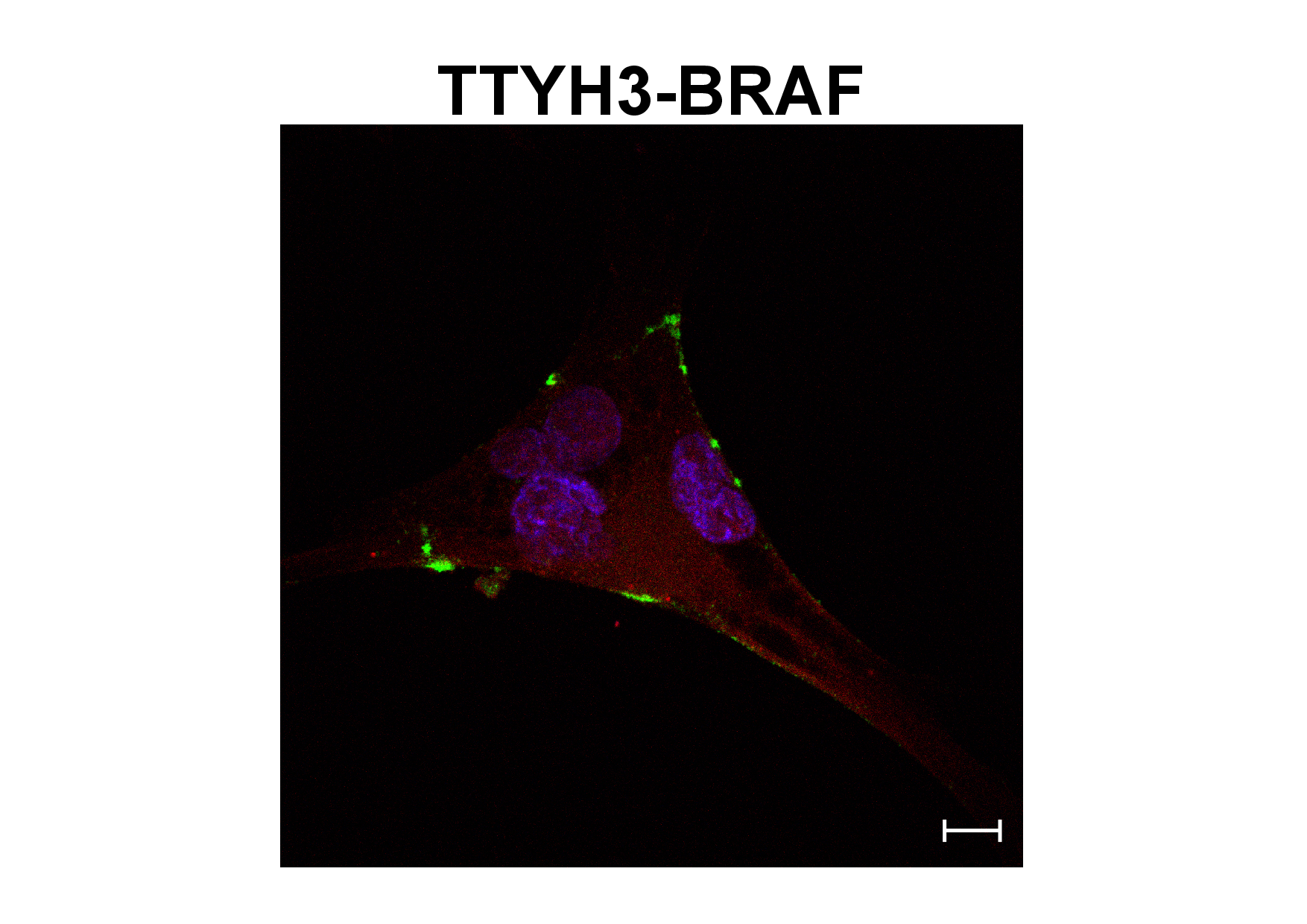
DKTK researchers and physicians from Freiburg and Heidelberg and from the NCT in Heidelberg have analyzed the complete genetic makeup of an aggressive brain tumor. The analysis was conducted within the NCT/DKTK MASTER trial. They discovered a new BRAF fusion protein, which has been named TTYH3-BRAF after its new genetic partner. BRAF kinase fusion proteins are created through spontaneous chromosomal shifts in the cancer cells that cause genes that are normally separate to join together. Their joint protein product acquires new characteristics. Its ability to drive the growth of aggressive tumors has been attributed to the loss of autoinhibitory domains of the BRAF protein.
However, a more detailed characterization of TTYH3-BRAF showed that all domains of the BRAF protein necessary for autoinhibition were still present. Despite this, the new fusion protein activated a BRAF-regulated signaling cascade – the MEK-ERK signaling pathway – and the cells under investigation developed into cancer cells in the lab. These effects are caused by the new TTYH3 region constantly tethering the near-full-length BRAF protein to the cell membrane, from where it can trigger the MEK-ERK signaling cascade, even in the absence of external growth signals.
“Our work describes for the first time a BRAF fusion protein with cancer-driving characteristics that are caused exclusively by the fusion partner and not by the defective structure of BRAF itself,” says Stefan Fröhling, interim Managing Director of NCT Heidelberg. In addition, the findings from the current research study show that future assessments of tumor-associated fusion proteins must also consider the characteristics of potential genetic fusion partners. “The defective signals emitted by the TTYH3-BRAF complex could be inhibited by active substances that have already been approved for cancer treatment or are being tested in clinical trials,” says Tilman Brummer, a researcher at the University of Freiburg. “That’s why our results provide information that is relevant for clinical application and which could influence treatment planning for patients.” Potential candidate substances for blocking the RAF-MEK-ERK signaling pathway include sorafenib and trametinib, two drugs that have already been approved for the treatment of other types of cancer.
The research was funded by the German Research Foundation (DFG).
Original publication
F. Weinberg, R. Griffin, M. Fröhlich, C.Heining, S. Braun, C. Spohr, M. Iconomou, V. Hollek, M. Röring, P. Horak, S. Kreutzfeldt, G. Warsow, B. Hutter, S. Uhrig, O. Neumann, D. Reuss, D. H. Heiland, C. von Kalle, W. Weichert, A. Stenzinger, B. Brors, H. Glimm, S. Fröhling, T. Brummer (2019) Identification and characterization of a BRAF fusion oncoprotein with retained autoinhibitory domains. Oncogene, doi: 10.1038/s41388-019-1021-1.